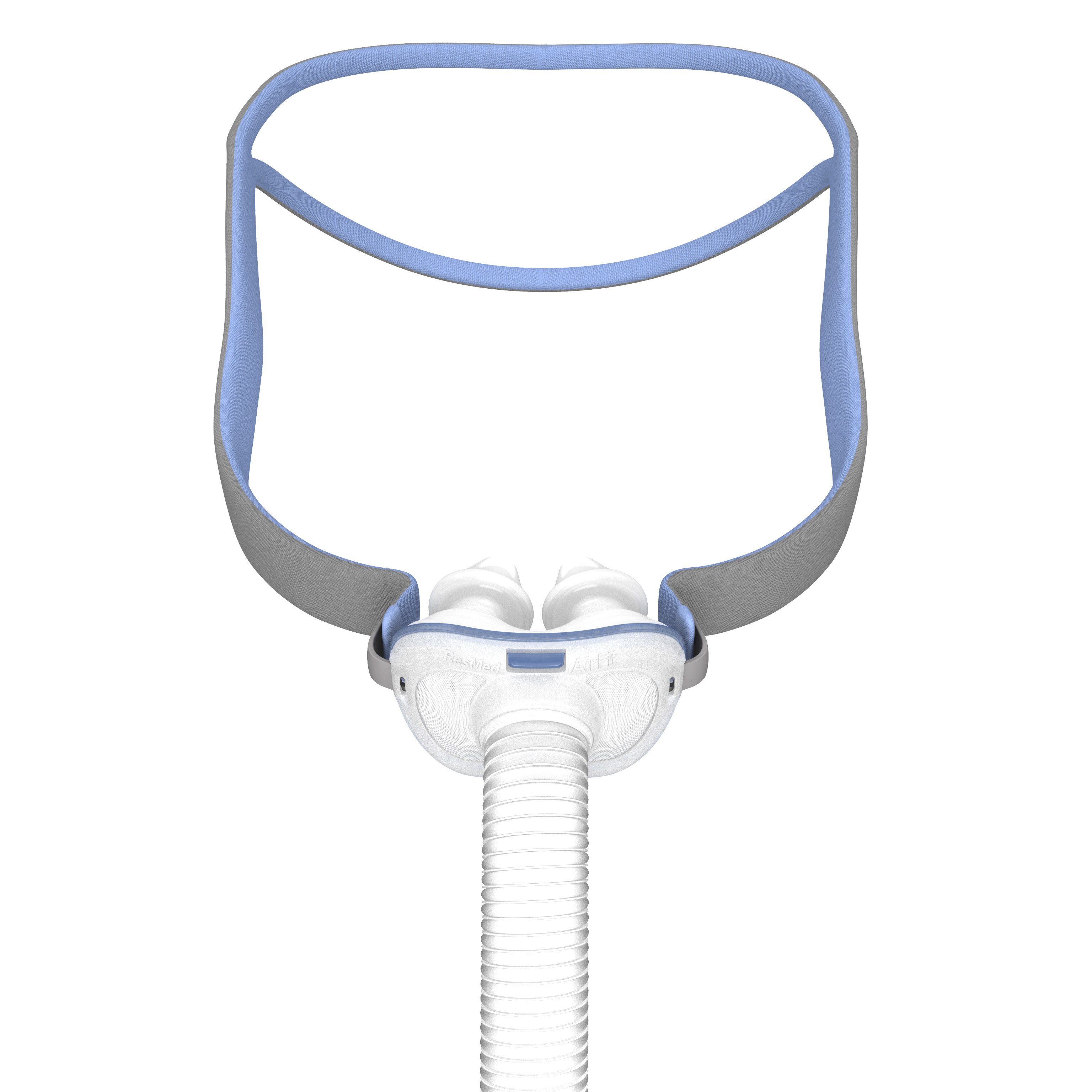
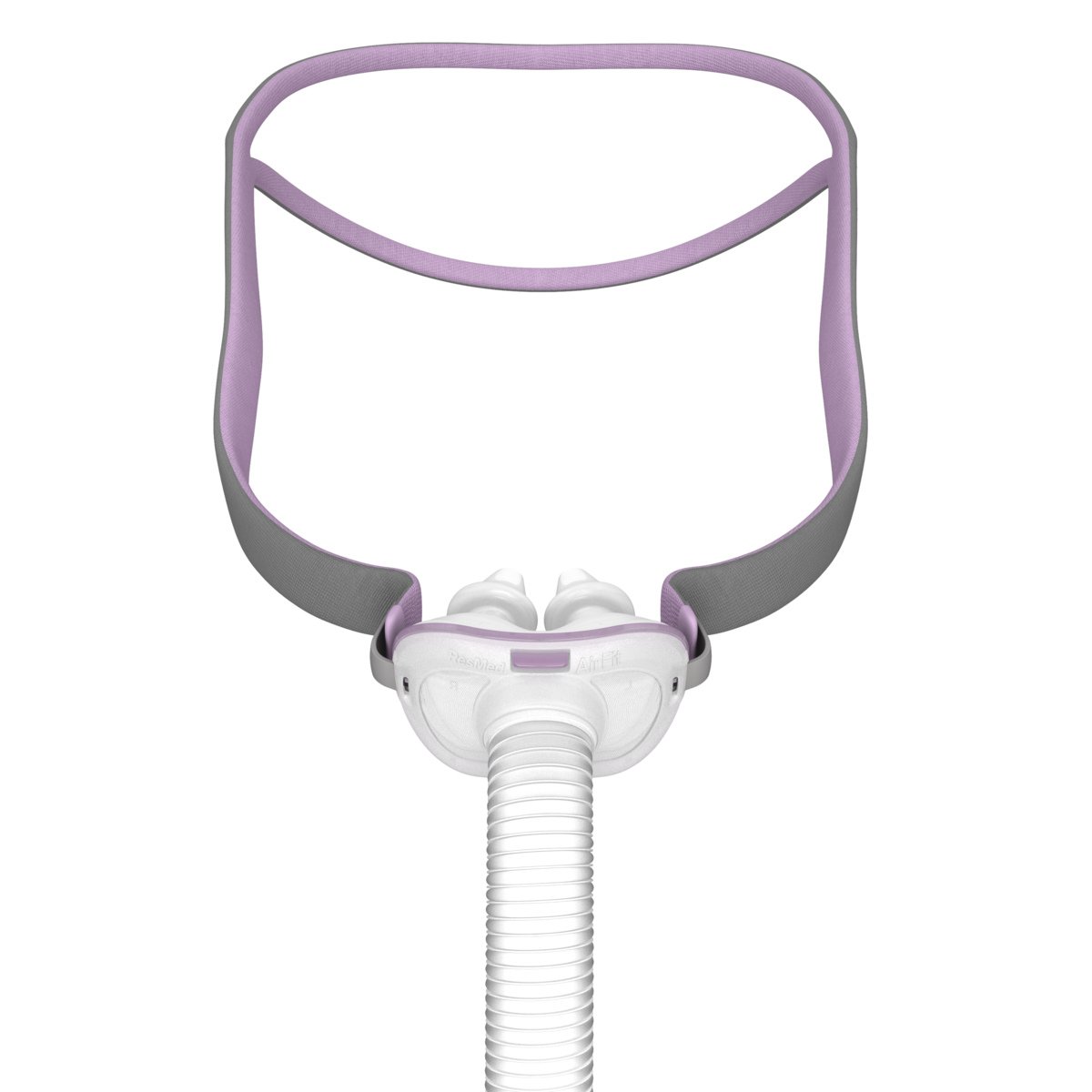
The AirFit™ F40 is a lightweight full face mask that sits softly below your nose, promoting comfortable sleep in any position and freedom of movement throughout the night.
This mask contains magnets that may intefere with certain implants on medical devices. Please see reference section below for complete labelling information, including magnet contraindications and warnings.
Choose between our physical dealers or online stores.

Registered under Act 737
Medical device registration number: GB6627624-173651
Advertisement approval number: MDAMD 0102/2025
I like to sleep on my side
I breathe through my mouth when I sleep
I wear glasses in bed
Contact your CPAP equipment provider—the store where you obtained your CPAP mask and machine—for ongoing clinical support. They can help with questions, resolve mask comfort or fitting issues, and explore different mask options that may better meet your personal comfort needs. Adjusting to a mask can be challenging, so don’t hesitate to seek assistance Not sure who your CPAP equipment provider is? We can help, fill out our Contact Us form or simply use our Chat Bot.
You should clean the cushion, elbow and short tube, and vent ring daily. The headgear and frame only require weekly cleaning. Facial oil and dirt can easily build up on your mask parts when they’re not cleaned properly, which can lead to skin irritation. The good news? Cleaning your mask doesn’t take long, so working it into your morning routine should be a breeze.
HOW TO CLEAN YOUR MASK
Supplies you’ll need to clean your mask:
Sink or tub
Warm, drinking-quality water (86°F / 30°C)
Mild liquid detergent
Towel
Step-by-step cleaning tips:
1. Unplug your CPAP machine from the power source and disconnect your mask/tubing from your machine.
2. Disassemble your mask:
- Undo the fastening tabs on the upper headgear straps and pull from the frame.
Tip: Keep the magnetic clips attached to the lower headgear straps to easily distinguish the upper and lower straps when reassembling.
- Squeeze the side buttons on the elbow and detach from the vent ring.
- Pull the cushion stud out of the frame stud hole, and repeat on the other side.
- Pull the vent ring from the cushion.
3. Soak the components in warm water with a mild liquid detergent. Ensure that there are no air bubbles while soaking.
4. Shake the components vigorously in the water and hand wash with a soft bristle brush. Pay particular attention to the vent holes in the vent ring.
5. Thoroughly rinse the components under running water.
6. Squeeze the fabric components to remove excess water.
7. Leave the components on the towel to air dry out of direct sunlight.
Refer to the product user guide for complete instructions.
Ozone or UV light products have not been authorized for mask cleaning. These products may cause discoloration or damage that is not covered under the ResMed limited warranty. Warranty coverage for mask issues is applicable only if they are observed upon initial unpackaging.
Regular maintenance of your CPAP equipment, including your mask, is critical to prevent discomfort and skin irritation from accumulated dirt and oil. Simplify your routine by inquiring with your CPAP equipment store about enrolling in a replacement supply program for timely access to new supplies.
Silicone is used in CPAP mask cushions because it's hypoallergenic, soft, lightweight, and provides a secure, comfortable seal during sleep. Your comfort and therapy adherence should influence your choice of mask cushion. If you have skin sensitivity or discomfort, reach out to your CPAP equipment store for support.
Having any difficulties? Talk to us so we can make your CPAP journey even better.
Source: Two ResMed external clinical studies of experienced PAP patients. The first study included users with ≥ 6 months’ therapy who used ResMed’s AirFit F40 and AirFit F20 full face masks in randomized order for up to seven consecutive nights each at home. Study conducted in Australia, June 6 – 27, 2023, n=28 (AirFit F40) and 29 (AirFit F20). Facial measurements of 29 participants were taken. The second study included current users with ≥ 12 months’ PAP therapy who met 90-day CMS compliance for PAP therapy in the three months prior to the study. Participants used ResMed’s AirFit F40 and Fisher & Paykel’s Evora Full in a randomized order with their own for up to seven consecutive nights each at home. Study conducted in the U.S., October 4 – November 20, 2023, n=57. Facial measurements of 57 participants were taken.
Source: Two ResMed external clinical studies of experienced PAP patients. The first study included users with ≥ 6 months’ therapy who used ResMed’s AirFit F40 and AirFit F20 full face masks in randomized order for up to seven consecutive nights each at home. Study conducted in Australia, June 6 – 27, 2023, n=28 (AirFit F40) and 29 (AirFit F20). Facial measurements of 29 participants were taken. The second study included current users with ≥ 12 months’ PAP therapy who met 90-day CMS compliance for PAP therapy in the three months prior to the study. Participants used ResMed’s AirFit F40 and Fisher & Paykel’s Evora Full in a randomized order with their own for up to seven consecutive nights each at home. Study conducted in the U.S., October 4 – November 20, 2023, n=57. Facial measurements of 57 participants were taken.
Source: Internal ResMed comparison of the ResMed AirFit F20, AirTouch F20, AirFit F30 and AirFit F40. Measurements are based on cushion mask on face, front view and side view.
Source: Testimonial from two ResMed external clinical studies of experienced PAP patients. The first study included users with ≥ 6 months’ therapy who used ResMed’s AirFit F40 and AirFit F20 full face masks in randomized order for up to seven consecutive nights each at home. Study conducted in Australia, June 6 – 27, 2023, n=28 (AirFit F40) and 29 (AirFit F20). Facial measurements of 29 participants were taken. The second study included current users with ≥ 12 months’ PAP therapy who met 90-day CMS compliance for PAP therapy in the three months prior to the study. Participants used ResMed’s AirFit F40 and Fisher & Paykel’s Evora Full in a randomized order with their own for up to seven consecutive nights each at home. Study conducted in the U.S., October 4 – November 20, 2023, n=57. Facial measurements of 57 participants were taken.